Technology Overview
Why do scale problems
occur?
1. Hard water contains too many mineral ions such as calcium and magnesium.
2.
Calcium carbonate is
less soluble in hot water. (Inverse solubility)

![]()
![]()
Solubility of
CaCO3
20ºC 50ºC 80ºC
3. As water enters the heat exchanger, the
solubility of CaCO3 drops. This is termed “Uncontrolled Precipitation.”
(Dissolved calcium ions)
![]() Ca++ + 2HCO3 CaCO3
+ H2CO3
Ca++ + 2HCO3 CaCO3
+ H2CO3
![]() Uncontrolled
Uncontrolled
Precipitation Scale Build-up
Calcium salts stick to metal surface due to
Electrostatic
attraction
Why is scaling a
problem?
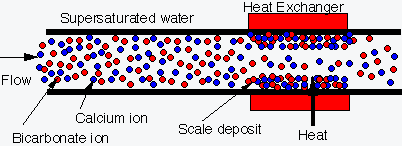
1. Scale layers act as a thermal insulator, decreasing efficiency of the heat exchanger. Why? A result of the small thermal conductivity of scale.
Calcium carbonate k = 0.8 (W/m K)
Silica k = 0.08
Copper k = 393
Steel k = 75
(Conversion: 1 W/m K = 0.5781
Btu/hr ft ºF)
2.
Narrowing of tube
opening
-
Flow rate through scaled tubes is significantly reduced.
What is ED 2000
Technology?
“Controlled
Precipitation”
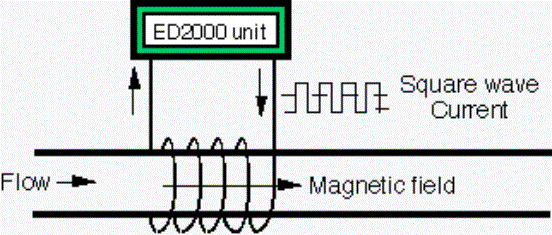
§
ED 2000 technology
uses a signal cable wrapped around the condenser or heat exchanger supply pipe.
§
An induced,
oscillating electric field is produced (known as Faraday’s Law).
The agitation of the
electrically charged mineral ions is caused by the electric field and the rapid
change of direction. The gauss strength
of this field is only one gauss and not to be confused with magnetic treatment.
(Refrigerator magnets have gauss strength of 1000 gauss. Magnetic treatment
requires both velocity and the gauss strength to cause the same mechanical
agitation.
Why is new scale
prevented?
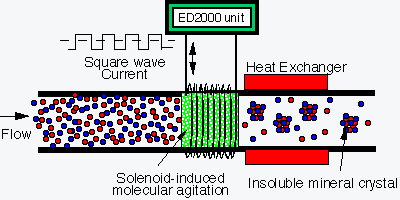
§
Solenoid-induced-molecular-agitation
(SIMA®) precipitates dissolved mineral ions to insoluble mineral
crystals.
§
These insoluble
crystals are suspended in the water and float with the water, but do not stick
to pipe walls and heat exchanger surfaces.
Specific gravity is greater that that of water but the flow velocity
keeps the crystals in suspension.
§
Hence, new scale is
prevented