|

Heat Exchange Unit
with scale buildup |
Problems
With Scale
Scale remains a serious problem to all industries that heat water in
their processes. It is especially true in plate and frame heat
exchangers.
|

Heat Exchange Unit
protected from scale
buildup by deposit control device |
Scale can build up on heat exchange surfaces, valves and pipes. The scale can
significantly reduce the heat transfer efficiency, cause valves to
stick, and clog pipes. Problems caused by scale include:
- Decreased energy efficiency
- Decreased flow rate in pipes
- Increased thermal resistance
- Increased downtime for cleaning
- Premature replacement of heat
exchange plates
- Oversized design to address scale
resistance
|
Patented
Technology
The ED2000
technology was developed and tested through a collaborative R&D
effort with Drexel University in Philadelphia, PA. The results
of the R&D effort are:
- Nine new patents
filed
- Perfected
technology to assure consistency
- Field tested with
major manufacturers
- Chemical-free,
environmentally friendly technology
|
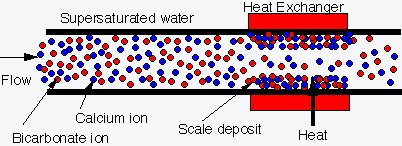
"Uncontrolled precipitation" as
scale precipitates onto heat exchanger surfaces |
- How does ED2000 electronic deposit control
technology work?
- This electronic deposit control method is based on
oscillating electrical field technology. The electronic deposit control technology uses a signal cable that
is wrapped around a pipe.
|
- The cable is connected to an electronic unit that sends a
complex, dynamic current with rapidly changing polarity to
produce an extremely small time-varying magnetic field inside the pipe (the magnetic field
is hundreds of times weaker than refrigerator magnets used in the kitchen).
-
- The time-varying magnetic field produces an induced,
oscillating electric field inside the pipe. The phenomenon is well-known as Faraday's
law. The induced, oscillating electric field provides the necessary molecular agitation
for scale prevention and removal.
|
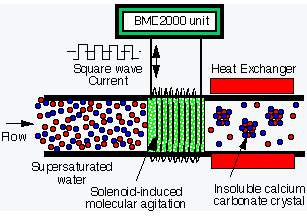
"Controlled
precipitation" converts dissolved mineral ions into
insoluble crystals, thus preventing scale formation on heat
exchanger surfaces |
Controlled
Precipitation
The key to
the ED2000 technology and its success relates to understanding water
chemistry and the dynamics of heat transfer equipment. The
ED2000 unit treats the water before it enters the heat transfer
area. The unit produces a solenoid-induced molecular agitation
to precipitate dissolved mineral ions to large insoluble mineral
crystals. By this "controlled precipitation" the
crystals suspend in the water and do not adhere to metal surfaces,
inhibiting the formation of scale. |
|
|
 |
Scanning
Electron Microscope Photographs taken with scale specimen from
scaled tubes produced at a flow velocity of 0.78 m s-1
and a concentration of 10 mol m-3 (a) without
electronic anti-fouling device and (b) with EAF device.
Magnification = 1500.
Scale specimen for SEM was
prepared with a utility knife by scraping small amounts of
scales from the outlet region of each scaled tube. SEM
photographs of scales produced without the EAF (electronic
anti-fouling) device (a) revealed that CaCO3 scales
were needle-shaped aragonite, whose dimensions were
approximately 25 mm by 2 mm. Aragonite is a dangerous form of
calcium carbonate scale, which is crystallized at a
temperature above 308 K. It is sticky, dense, and difficult to
remove. The long-needle shaped crystals confirm that the
precipitation reaction occurred on the heat transfer surface
without the EAF device.
n
contrast, the scales produced with the EAF device (b) depicted
a very different structure from the one produced without the
EAF device. The scales produced with the EAF device were a
cluster of small elliptic shape particles (e.g., 10 mm by 3
mm) with no particular orientation, suggesting that many fine
particles were formed in bulk solution, attached to the heat
transfer surface, and then grew in size through precipitation
reaction.
|
The SEM photographs support the
hypothesis of the EAF technology, which is to convert
dissolved mineral ions into crystals in a bulk solution, thus
reducing both the diffusion of dissolved ions to the heat
transfer surface and subsequent precipitation reaction on the
heat transfer surface. As a result, the production of
aragonite type calcium carbonate is prevented. Calcium
carbonate crystals formed with the EAF device appeared to be
loosely connected. In other words, the scales formed with the
EAF device may be removed at a small flow velocity (e.g., 1 m
s-1), whereas the scales formed without the EAF
device may not be removed even at a large flow velocity (e.g.,
5 m s-1). |
|
When the ED2000 treats
water or other fluids, several things happen.
- The hydrogen
bonds between water molecules are broken and more water molecules are freed to hydrate
scale ions and colloidal particles.
- The scale ions dissolved in the water are agitated, they collide,
and form scale molecules that join together to form crystals.
- The colloidal particles in the water receive an enhanced surface
charge, possibly from the freed water molecules. The enhanced surface charge is great
enough for the colloidal particles to repel each other and the sides of the equipment, and
stay suspended in the fluid.
|
|