
Quick Index:
Fouling Laboratory at Drexel University has been conducting research to understand the basic fouling mechanism occurring in various heat exchangers and to control fouling. In order to control the fouling, the Fouling Laboratory has developed a new innovative technology called Electronic Descaling (ED) technology.
Background
When hard water is heated (or cooled) in heat transfer equipment, scaling occurs. When scales deposit on a heat exchanger surface, it is traditionally called "fouling". The type of scale differs from industry to industry, depending on the mineral content of the available water. One of the most common forms of scale is calcium carbonate, CaCO3, which is the subject of the present study. Other mineral salts such as calcium phosphate, barium carbonate, silica and others have been tested and are successfully treated through the ED process.


1. Scale layers act as thermal insulator, decreasing efficiency of HX.
Why? Small thermal conductivity of
scales calcium carbonate:
k = 0.8 (W/m K)
silica k = 0.08
copper k = 393
steel k = 75
(Conversion: 1 W/m K = 0.5781 Btu/hr ft oF)
* Oversized heat exchanger.
2. Narrowing of tube opening
B- Flow rate through scaled tubes is significantly reduced.
Controlled Precipitation
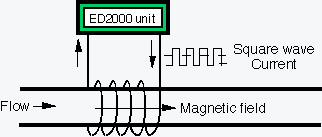
ED technology uses a solenoid cable that is wrapped around a pipe.
Square wave current signal generates time-varying magnetic field inside solenoid. Subsequently, the time-varying magnetic field creates an induced electric field inside the pipe, a phenomenon which can be described by Faraday's law:
The induced electric field, which oscillates with time, provides molecular agitation to charged mineral ions such that dissolved mineral ions such as calcium and bicarbonate collide and precipitate with the help of impurities in water (e.g., iron oxide particles).
Ca+2 + 2HCO3- -----> CaCO3 + H2CO3

Solenoid-Induced-molecular-agitation (SIMAĆ) precipitates dissolved mineral ions to insoluble mineral crystals. Hence, new scale is prevented.
Before ED treatment
After ED treatment

Sketch
of cross section of a pipe 
(a) for E field in the clockwise direction, positive ions move clockwise while negative ions move counterclockwise, resulting in collision and precipitation in bulk solution and (b) for E field in the counterclockwise direction, positive ions move counterclockwise while negative ions move clockwise.
Before Treatment

After Treatment

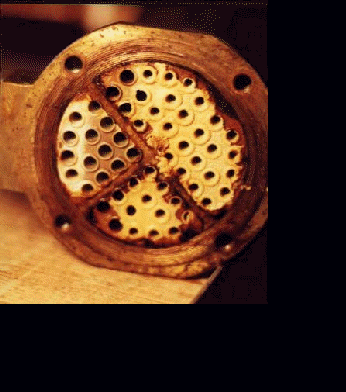
Scanning Electron Microscope Photographs taken with scale specimen from scaled tubes produced at a flow velocity of 0.78 m s-1 and a concentration of 10 mol m-3 (a) without electronic anti-fouling device and (b) with EAF device. Magnification = 1500.

Scale specimen for SEM was prepared with a utility knife by scraping small amounts of scales from the outlet region of each scaled tube. SEM photographs of scales produced without the EAF (electronic anti-fouling) device (a) revealed that CaCO3 scales were needle-shaped aragonite, whose dimensions were approximately 25 mm by 2 mm. Aragonite is a dangerous form of calcium carbonate scale, which is crystallized at a temperature above 308 K. It is sticky, dense, and difficult to remove. The long-needle shaped crystals confirm that the precipitation reaction occurred on the heat transfer surface without the EAF device.
In contrast, the scales produced with the EAF device (b) depicted a very different structure from the one produced without the EAF device. The scales produced with the EAF device were a cluster of small elliptic shape particles (e.g., 10 mm by 3 mm) with no particular orientation, suggesting that many fine particles were formed in bulk solution, attached to the heat transfer surface, and then grew in size through precipitation reaction.
The SEM photographs support the hypothesis of the EAF technology, which is to convert dissolved mineral ions into crystals in a bulk solution, thus reducing both the diffusion of dissolved ions to the heat transfer surface and subsequent precipitation reaction on the heat transfer surface. As a result, the production of aragonite type calcium carbonate is prevented. Calcium carbonate crystals formed with the EAF device appeared to be loosely connected. In other words, the scales formed with the EAF device may be removed at a small flow velocity (e.g., 1 m s-1), whereas the scales formed without the EAF device may not be removed even at a large flow velocity (e.g., 5 m s-1).
How is ED2000 different from other solutions?
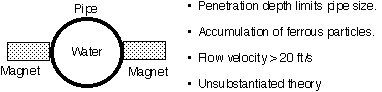
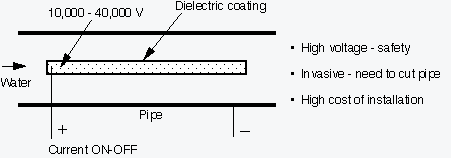
How? Discharge water contains more mineral crystals, reducing TDS level in a recirculating loop, thus saving water.
[
Top of
Page |
Back
to Case Studies |
Return
to Home
]
Effect of pressure change on scaling in pump and valve
[ Top of Page | Back to Case Studies | Return to Home ]