Validation of Electronic Anti-Fouling Technology in a Single-Tube Heat Exchanger
The objective of the present study was to investigate the validity of an electronic anti-fouling (EAF) technology in the mitigation of precipitation fouling in a once-through flow system with a single-tube heat exchanger. Effects of flow velocity and water hardness on the effectiveness of the EAF technology were experimentally studied. The water hardness varied from 750 to 1,000 mg/L as CaCO3. For both 750 and 1,000 mg/L solutions, the EAF treatment reduced fouling resistance by 20-38 % at a flow velocity between 0.52 and 0.78 m/s. As the velocity decreased to 0.28 m/s, the EAF treatment was not helpful in reducing fouling. SEM photographs of scale produced from the 1,000 mg/L solution at 0.78 m/s indicated that calcium carbonate scales without the EAF treatment were needle-shaped aragonite, which is sticky, dense, and difficult to remove. Scales with the EAF treatment showed a cluster of elliptic shape crystals. Both the heat transfer test results and SEM photographs obtained in the present study support the validity of the EAF technology in mitigating precipitation fouling.
Nomenclature
A = heat transfer surface area
A = cross sectional area vector
B = magnetic field strength vector
dt,o = outside tube diameter
DH
= hydraulic diameter
Ds
= inside shell diameter
E = induced electric field intensity vector
Q
= heat transfer rate
s = line vector along the circumferential direction
Tc,in = inlet temperature of cold water
Tc,out
= outlet
temperature of cold water
DTLMTD = log-mean-temperature-difference
U = overall heat transfer coefficient based on outside tube diameter
Uclean = overall heat transfer coefficient at clean state
Experimental
Method
Figure
2 schematically shows the test facility which consists of two reservoir tanks,
two pumps, two flow meters, a static mixer, an electronic anti-fouling control
unit, a solenoid coil, the main heat transfer test section made of a
shell-and-tube heat exchanger, and a plate-and-frame heat exchanger.
Most previous fouling studies [8-13] were conducted using a recirculating flow system. The critical drawback of conducting a fouling test using a recirculating system is the continuous reduction of hardness during the test. Indeed, most fouling problems in the real world occur in a once-through flow system. In order to create the best simulation of fouling problems occurring in the real world the present study conducted fouling test in a once-through flow system.
The
main heat transfer test section is made of two concentric copper tubes, which
form a counter-flow heat exchanger. Two
reducing unions were used at both the inlet and outlet of the heat exchanger
for easy connection between the heat exchanger and connecting pipes.
Hard (cold) water was pumped to the shell side whereas hot water moved
through the tube side. Hence, scales were deposited on the shell side only.
The shell diameter (ID) was Ds
= 0.01684 m whereas the tube diameter (OD) was dt,o
= 0.01275 m, rendering the hydraulic diameter of DH
= 0.00409 m. The
diameter of both the inlet and outlet connecting tubes was 0.0109 m. The axial length of the tube in the heat exchanger was 0.7 m.
Since
the hardness of tap water available in Philadelphia is approximately 150 mg/L
as CaCO3, it is not suitable
for fouling experiments. Therefore,
hard water in a range of 750 to 1,000 mg/L was prepared in a laboratory.
For example, in order to prepare 1,000 mg/L CaCO3
solution, 0.01 M calcium chloride (CaCl2)
and 0.02 M sodium bicarbonate (NaHCO3)
were added to tap water. Table 1
gives values of alkalinity, electric conductivity, and pH of each
concentration solution. The
resulting hard water caused severe scaling inside a pump, and one pump was
lost during almost every test due to the pump scaling.
In order to avoid the scaling in the pump, the calcium chloride and
sodium bicarbonate solutions were prepared in two separate reservoir tanks,
pumped using two separate pumps and two flow meters, and later mixed using a
static mixer as shown in Fig. 2.
The
inlet temperature of the hard water was maintained at 302±0.5oK
for all tests. Flow rates varied
from 2.71 x10-5 m3/s
(0.43 gpm) to 7.57x10-5 m3/s
(1.2 gpm), which resulted in the shell-side velocity of 0.28 and 0.78 m/s,
respectively. The Reynolds
numbers corresponding to 0.28 and 0.78 m/s were 1,870 and 5,020, respectively. It is of note that the viscosity and density of the hard
water used in the Reynolds number calculation were evaluated at an average
temperature of the hard water.
Thermocouples
used in the present study were Omega model TMTSS-125G-6 (grounded
copper-constantan T type). Calibration
was carried out at 273 and 373oK,
confirming the manufacturer's claim of the accuracy of ±0.1oK.
The temperature of the hot stream entering the tube side varied from
355 to 367±1.0oK in order to
study the effect of hot water temperature on the fouling resistance and
initial fouling rate. To produce
hot water continuously, a plate-and-frame heat exchanger was used, where high
pressure steam provided by the Philadelphia city steam network was used.
Flow meters were also calibrated over a range of 6.3 x10-6
to 6.3 x10-5 m3/s
by measuring the weight of water accumulated over time, and the calibration
results are reported elsewhere [14].
The overall heat transfer coefficient, U, was calculated as a function of time using the log-mean-temperature-difference (LMTD) [15], which was obtained from the four temperatures measured at both the inlet and outlet of cold and hot streams. Fouling resistance, Rf, was calculated using the usual definition [15].
Q = U A DT = Cp (Tc,out - Tc,in) (3)
U = (4)
and
Rf = - (5)
where
Uclean is
the overall heat transfer coefficient corresponding to the clean state, i.e.,
at t = 0. The Uclean
values for 0.28, 0.52 and 0.78 m/s cases were 2,460, 3,430, and 4,130 W/m2K,
respectively.
The
errors estimated from the uncertainty analysis for Q, U, Rf,
and A (heat transfer surface area) were 2.5%, 3.0%, 8.0%, and
1.1%, respectively. During the
course of the present fouling experiment, it was discovered that the accuracy
of the outlet temperature reading could be significantly affected by the
scaling of the thermocouple probe itself [16].
Therefore, the thermocouple measuring the outlet temperature of the
cold water was cleaned every time before each temperature measurement.
It is speculated that much of the previous fouling data in the
literature might have been seriously affected by the probe fouling because the
thermocouple fouling could easily cause a temperature measurement error by
several degrees.
Results
and Discussion
Figure 3
represents variation in fouling resistance as a function of time at a flow
velocity of 0.78 m/s (1.2 gpm) and a concentration of 1,000 mg/L with and
without electronic anti-fouling (EAF) device.
The hot water inlet temperature was 367oK for both cases, and the flow rate of
hot water was 2.23 m/s (3.3 gpm). The
fouling resistance for both cases, as manifested by the slopes in Fig. 3,
increased linearly with time, indicating that the fouling was mostly
controlled by a diffusion mechanism.
From the
slope of the curve of Rf
vs. time in Fig. 3, the fouling rate without the EAF device was 1.45x10-6
m2K/J, whereas the value with
the EAF device was 1.15x10-6 m2K/J.
At the end of 86 min. of operation, the fouling resistance without the
EAF device was 0.0001287 m2K/W,
whereas it was 0.0001036 m2K/W
with the EAF device, a 20% drop.
It was
hypothesized that the EAF treatment produced
CaCO3 particles, reducing the
mass transfer coefficient of calcium ions and subsequently the scale deposit
rate. In order to examine this
hypothesis, a scanning electron microscopy (SEM) was used to visualize crystal
structures of scales. SEM
photographs given in Figs. 4a and 4b were obtained from scaled tubes produced
at a flow velocity of 0.78 m/s (1.2 gpm) and a concentration of 1,000 mg/L
without and with EAF device, respectively. SEM photographs taken using specimen from other scale tubes
obtained under different conditions are given elsewhere [14].
Scale
specimen for SEM was prepared with a utility knife by scraping small amounts
of scales from the outlet region of each scaled tube.
SEM photographs of scales produced without the EAF device (see Fig. 4a)
revealed that CaCO3 scales
were needle-shaped aragonite [17], whose dimension was approximately 25 mm
by 2 mm.
Aragonite is a dangerous form of calcium carbonate scale, which is
crystallized at a temperature above 303oK
[17]. It is sticky, dense, and
difficult to remove.
In contrast,
the scales produced with the EAF device (see Fig. 4b) depicted a very
different structure from the one produced without the EAF device.
The scales produced with the EAF device were a cluster of small
elliptic shape particles (e.g., 10 mm by 3 mm) with no
particular orientation, which are much easier to remove than aragonite.
The SEM photographs in Fig. 4 support the hypothesis of the EAF
technology, which is to convert dissolved mineral ions into crystals in a bulk
solution, thus reducing both the diffusion of dissolved ions to the heat
transfer surface and subsequent precipitation reaction on the heat transfer
surface. As a result, the
production of aragonite type calcium carbonate is prevented.
Calcium carbonate crystals formed with the EAF device appeared to be
loosely connected compared to the one formed without it.
In other words, the scales formed with the EAF device may be removed at
a small flow velocity (e.g., 2 m/s), whereas the scales formed without the EAF
device may not be removed even at a large flow velocity (e.g., 5 m/s).
The determination of the exact threshold flow velocity for both cases
is currently under way and will be reported in the future.
Figure 5
represents variation in fouling resistance as a function of time at a flow
velocity of 0.52 m/s (0.8 gpm) and a concentration of 1,000 mg/L with and
without EAF device. The hot water
inlet temperature maintained to 361oK for both cases, and the flow rate of hot water was 0.74
m/s (1.1 gpm). In spite of a
reduced flow velocity of cold water (i.e., 0.52 m/s), the fouling resistance
leveled off after approximately 90 min. of operation, reaching asymptotic
fouling resistances of 0.0001287 m2K/W and 0.0001036 m2K/W for the cases without and with the
EAF device, respectively. The
asymptotic fouling resistance obtained with the EAF device was 23% less than
that without the EAF device.
Figure 6
shows variation of fouling resistance as a function of operating time at a
cold water flow velocity of 0.28 m/s and a concentration of 1,000 mg/L with
and without EAF device. The hot water inlet temperature was 367oK for both cases, and the flow
rate of hot water was 2.23 m/s - a severe fouling condition.
At the end of 240 min. of operation, the fouling resistance obtained
with the EAF device was almost identical to the one obtained without the
device. The EAF treatment at this
reduced flow velocity was found to be not much useful in mitigating fouling.
In order to
understand why the EAF treatment did not reduce fouling under these severe
fouling condition, one can examine the fouling curve in Fig. 6.
Initial fouling rate without the EAF device was greater than that with
the EAF device (i.e., see the slopes of the fouling curves at t = 0).
Hence, more scales deposit on tube surface without the EAF device,
reducing scale surface temperature. Subsequently,
less scales deposit due to the decrease in the surface temperature, as
indicated by arrow “A” in Fig. 6. On
the other hand, less scales deposit initially with the EAF device.
Subsequently, the temperature drop in the heat transfer surface is
less, and fouling rapidly progresses as indicated by arrow “B” in Fig. 6.
As enough scales deposit on the tube surface eventually, the surface
temperature drops, leading to asymptotic fouling resistance.
Figure 7 presents variation in
fouling resistance as a function of time at a flow velocity of 0.78 m/s (1.2
gpm) and a concentration of 750 mg/L with and without EAF device.
The hot water inlet temperature was 94oC
for both cases, and the flow rate of hot water was 2.23 m/s. At the end of 89 minutes of operation, the fouling resistance
without the EAF device was 5.086x10-5 m2K/W, whereas the value with the EAF
device was 3.78x10-5
m2K/W,
a 26% drop. Compared with the
results given in Fig. 3, the percentage drop in the fouling resistance due to
the EAF device was greater for the 750 mg/L solution than for the 1,000 mg/L
solution, suggesting that as a fouling condition becomes less severe the
benefit of the EAF treatment may become large.
In order to verify this
suggestion, the inlet temperature of hot water was reduced from 367oK
to 355oK,
and the corresponding test results obtained with and without the EAF device
are shown in Fig. 8. The flow
velocities of cold and hot water were 0.65 and 2.23 m/s (1.0 and 3.3 gpm),
respectively. At the end of 105
min. of operation, the fouling resistance without the EAF device was 5.25x10-5
m2K/W, whereas the value with
the EAF device was 3.26x10-5 m2K/W,
a 38% drop. As the hardness of
cold water decreased from 1,000 to 750 mg/L, the percentage drop in the
fouling resistance due to the EAF device increased from 20 to 38%.
Of note is that the hardness of cooling tower water is usually
maintained at an electric conductivity between 1,200 and 1,500 mS/cm,
which is equivalent to a hardness of approximately 450-500 mg/L.
Hence, the EAF treatment should reduce the fouling resistance in a heat
exchanger used in any cooling tower system by at least 40%.
Figure 8 also shows a peculiar
fouling behavior during the first 20 minutes of operation for both cases with
and without the EAF device. As
soon as tests started, the fouling resistance became negative for both cases,
indicating that the overall heat transfer coefficient, U, increased from that
of the clean state. This peculiar
phenomenon has not been uncommon [18]. This
can be explained as follows: The
nascent fouling deposit produces a roughened heat transfer surface.
As the surface becomes rough, the convective heat transfer coefficient
increases through better mixing and breaking the laminar sublayer at the wall.
Hence, the overall heat transfer coefficient initially improved,
resulting in negative fouling resistances.
As fouling deposits continue, the fouling layer acts as a thermal
insulator, decreasing the overall heat transfer coefficient.
The fouling resistance begins to increase and becomes positive at t =
13 min. without the EAF device and at t = 23 min. with the EAF device.
The trend of the fouling resistance having negative values initially
must have occurred for other tests whose results are shown in Figs. 3-6. It is speculated that this period of the heat transfer
improvement was too brief to observe.
Conclusions
The
present study was conducted in order to investigate the validity of electronic
anti-fouling (EAF) technology, which was considered as a means to control new
precipitation fouling in a heat exchanger.
Fouling tests were carried out in a counter-flow shell-and-tube heat
exchanger, and the fouling resistances obtained without the EAF device was
compared with those obtained the EAF device for two different water hardness
(750 and 1,000 mg/L) and three different flow velocities (0.28, 0.52 and 0.78
m/s).
Scanning
electron microscopy (SEM) photographs of scale produced without the EAF device
revealed needle-shaped aragonite (i.e., sticky, dense, and difficult to
remove), whereas the scale produced with the EAF device produced a cluster of
loosely-connected small elliptic shape particles (i.e., easier to remove than
aragonite).
For
the 1,000 mg/L solution at a velocity of 0.78 m/s, the fouling resistance with
the EAF device was 20% smaller than that without it. As the hardness of water decreased from 1,000 to 750 mg/L,
the percentage drop in the fouling resistance due to the EAF device increased
from 20 to 38%. However, at a reduced flow velocity at 0.28 m/s or less, the
EAF treatment was not helpful in the mitigation of fouling.
Although
the present paper uses calcium carbonate as an example of the mineral scales,
the electronic anti-fouling treatment is not limited to the calcium carbonate
scale. This is because the EAF
treatment utilizes the electrical charges of dissolved ions to foster
collision and precipitation into insoluble crystals.
References
1.
Tchobanoglous, G. and Burton, F., Wastewater
Engineering, Treatment, Disposal and Reuse, 3rd edn., McGraw-Hill, New
York, 1991.
2.
Serway, R.A., Physics for Scientists and Engineers, 3rd ed., Saunders College
Publishing, Philadelphia, PA, 1990, p. 874-891.
3. Fan, C.F., A Study of Electronic Descaling Technology to
Control Precipitation Fouling. Ph.D. thesis, Drexel University, Philadelphia,
PA, 1997.
4. Fan, C.F. and Cho, Y. I., Microscopic Observation of Calcium Carbonate Particles: Validation of an Electronic Anti-Fouling Technology. Int. Comm. Heat Mass Transfer, 1997, 24, 757-770.
5.
Fan, C.F. and Cho, Y. I.,
A New Electronic Anti-Fouling Method to Control Fouling. National
Heat Transfer Conference, Vol. 12.
Baltimore, Maryland, 1997, p. 183-188.
6. Cho, Y. I.,
Fan, C.F., and Choi, B.G., Theory of Electronic Anti-Fouling Technology to
Control Precipitation Fouling in Heat Exchangers. Int.
Comm. Heat Mass Transfer, 1997, 24,
747-756.
7. Cho, Y. I., Choi, B.G., and Drazner, B.J., Use of Electronic Descaling Technology to Control Precipitation Fouling in Plate-and-Frame Heat Exchanger. Compact Heat Exchangers for the Process Industries, (ed. by R.K. Shah), Begell House, New York, 1997, p. 267-273.
8. Hasson, D., Avriel, M., Resnick, W., Rozeman, T., and Windreich, S., Mechanism of Calcium Carbonate Scale Deposition on Heat Transfer Surfaces, Int. Eng. Chem. Fund., Vol. 7, 1968, p. 59-65.
9. Sheikholeslami, R. and Watkinson, A.P., Scaling of Plain and Externally Finned Heat Exchanger Tubes, J. of Heat Transfer, Vol. 108, 1986, p. 147-152.
10. Watkinson, A.P. and Martinez, O., Scaling of Heat Exchanger Tubes by Calcium Carbonate, J. of Heat Transfer, 1975, p. 504-508.
11. Watkinson, A.P., Water Quality Effects on Fouling from Hard Waters, in: Heat Exchangers - Theory and Practice, Taborek, J. et al., Hemisphere, 1983, p. 853-861.
12. Andritsos, N., Kontopoulou, M., and Karabelas, A.J., Calcium Carbonate Deposit Formation under Isothermal Conditions, Can. J. Chem. Eng., Vol. 74, 1996, p.911-919.
13. Rabas, T.J. and Tarborek, J., 1996, Heat-Rate Improvements Obtained by Retubing Condensers with New, Enhanced Tube Types, J. of Enhanced Heat Transfer, Vol. 3, p. 83-94.
14. Choi, B.G., A Study of Fouling Control in Heat Exchangers with Electronic Anti- Fouling Technology, Ph.D. thesis, Drexel University, Philadelphia, PA, 1998.
15. Incropera, F. P. and DeWitt, D.P., Fundamentals of Heat and Mass Transfer, 4th Edition., Wiley, New York, 1996.
16. Cho, Y.I., and Choi, B.G., Effect of Fouling on Temperature Measurements Error and a Solution, J. of Heat Transfer (in press).
17. Cowan, J.C., Weintritt, D.J., Water-Formed Scale Deposits, Gulf Publishing Company, Houston, TX, 1976.
18.
Bansal, B., and M
![]() ller-Steinhagen, Crystallization Fouling in Plate Heat Exchangers, J.
of Heat Transfer, Vol. 115, 1993, p. 584-591.
ller-Steinhagen, Crystallization Fouling in Plate Heat Exchangers, J.
of Heat Transfer, Vol. 115, 1993, p. 584-591.
Table 1 Values of alkalinity, electric conductivity, and pH of each concentration solution. Also shown are the amounts of calcium chloride CaCl2 and sodium bicarbonate NaHCO3 to tap water for 750 and 1,000 mg/L solutions.
|
Concentration (mg/L) |
CaCl2 (mol) |
NaHCO3 (mol) |
Alkalinity (mg/L) |
Conductivity (µS/cm) |
pH |
|
750 |
0.0075 |
0.015 |
760-810 |
3,060-3,190 |
7.88-8.10 |
|
1,000 |
0.01 |
0.02 |
980-1,040 |
3,860-3,950 |
7.81-8.05 |
|
|
|
|
|
|
|
List of Figures
Fig.
1 Schematic diagram of the
concept of electronic anti-fouling (EAF) technology.
EAF control unit produces a solenoid-induced molecular agitation
through Faraday’s law and is installed in a feed pipe prior to heat
exchanger.
Fig 2. Schematic diagram of a once-through flow system with a counter-flow single-tube heat exchanger. An EAF solenoid coil is located between pump and the inlet of the main heat transfer test section.
Fig. 3 Fouling factors vs. time for two different cases: without and with electronic anti-fouling (EAF) device. Concentration of test solution = 1,000 ppm; cold water flow velocity = 0.78 m/s; hot water flow velocity = 2.23 m/s.
Fig. 4 Photographs taken using a scanning electron microscopy with scale specimen from scaled tubes produced at a flow velocity of 0.78 m/s and a concentration of 1,000 mg/L (a) without electronic anti-fouling device and (b) with EAF device. Magnification = 1,500.
Fig. 5 Fouling factors vs. time for two different cases: without and with electronic anti-fouling (EAF) device. Concentration of test solution = 1,000 ppm; cold water flow velocity = 0.52 m/s; hot water flow velocity = 0.74 m/s.
Fig. 6 Fouling factors vs. time for two different cases: without and with electronic anti-fouling (EAF) device. Concentration of test solution = 1,000 ppm; cold water flow velocity = 0.28 m/s; hot water flow velocity = 2.23 m/s.
Fig. 7 Fouling factors vs. time for two different cases: without and with electronic anti-fouling (EAF) device. Concentration of test solution = 750 ppm; cold water flow velocity = 0.78 m/s; hot water flow velocity = 2.23 m/s.
Fig. 8 Fouling factors vs. time for two different cases: without and with electronic anti-fouling (EAF) device. Concentration of test solution = 750 ppm; cold water flow velocity = 0.78 m/s; hot water flow velocity = 2.23 m/s.
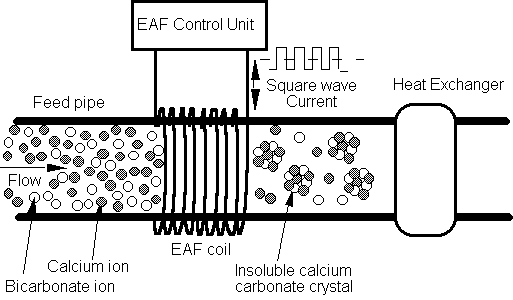
Fig. 1
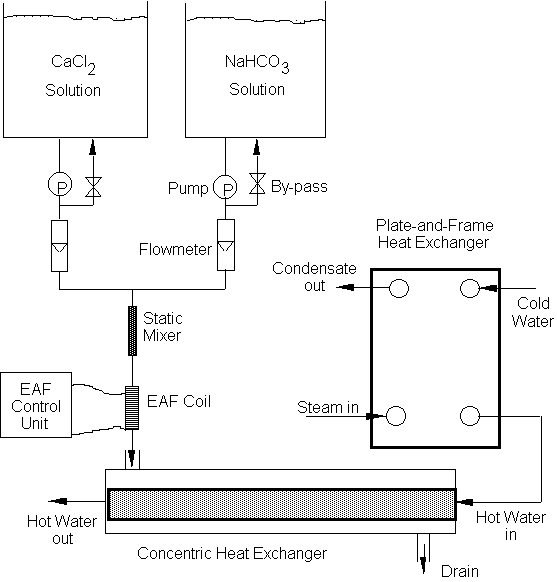
Fig. 2